Para ser un cientifico no basta solo con tener un pensamiento racional y dominar el metodo cientifico y la Metologia de la Investigacion. Son necesarios tambien valores Morales como la Honestidad y el respeto por la verdad.

lunes, 14 de marzo de 2011
Vacante en HEJCU
Saludos Colegas nuevamente les aviso que se esta convocando una plaza mas a un medico Internista en el HEJCU como CAS 3,500
Jackson Delgado
nuevas drogas para cancer de pulmon
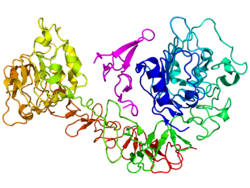
Epidermal growth factor receptor
| edit |
The epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans) is the cell-surface receptor for members of the epidermal growth factor family (EGF-family) of extracellular protein ligands.[1] The epidermal growth factor receptor is a member of the ErbB family of receptors, a subfamily of four closely related receptor tyrosine kinases: EGFR (ErbB-1), HER2/c-neu (ErbB-2), Her 3 (ErbB-3) and Her 4 (ErbB-4). Mutations affecting EGFR expression or activity could result in cancer.[2] Epidermal Growth Factor was discovered by Stanley Cohen of Vanderbilt University along with Rita Levi-Montalcini for which both received the Nobel prize in Physiology or Medicine in 1986.
| |
[edit] Function
EGFR (epidermal growth factor receptor) exists on the cell surface and is activated by binding of its specific ligands, including epidermal growth factor and transforming growth factor α (TGFα) (note, a full list of the ligands able to activate EGFR and other members of the ErbB family is given in the ErbB article). ErbB2 has no known direct activating ligand, and may be in an activated state constitutively or become active upon heterodimerization with other family members such as EGFR. Upon activation by its growth factor ligands, EGFR undergoes a transition from an inactive monomeric form to an active homodimer - although there is some evidence that preformed inactive dimers may also exist before ligand binding[citation needed]. In addition to forming homodimers after ligand binding, EGFR may pair with another member of the ErbB receptor family, such as ErbB2/Her2/neu, to create an activated heterodimer. There is also evidence to suggest that clusters of activated EGFRs form, although it remains unclear whether this clustering is important for activation itself or occurs subsequent to activation of individual dimers[citation needed].
EGFR dimerization stimulates its intrinsic intracellular protein-tyrosine kinase activity. As a result, autophosphorylation of several tyrosine (Y) residues in the C-terminal domain of EGFR occurs. These include Y992, Y1045, Y1068, Y1148 and Y1173 as shown in the diagram to the left.[3] This autophosphorylation elicits downstream activation and signaling by several other proteins that associate with the phosphorylated tyrosines through their own phosphotyrosine-binding SH2 domains. These downstream signaling proteins initiate several signal transduction cascades, principally the MAPK, Akt and JNK pathways, leading to DNA synthesis and cell proliferation.[4] Such proteins modulate phenotypes such as cell migration, adhesion, and proliferation. Activation of the receptor is important for the innate immune response in human skin.[5] The kinase domain of EGFR can also cross-phosphorylate tyrosine residues of other receptors it is aggregated with, and can itself be activated in that manner.;'
[edit] Clinical applications
Mutations that lead to EGFR overexpression (known as upregulation) or overactivity have been associated with a number of cancers, including lung cancer, anal cancers[6] and glioblastoma multiforme. In this latter case a more or less specific mutation of EGFR, called EGFRvIII is often observed.[7] Mutations, amplifications or misregulations of EGFR or family members are implicated in about 30% of all epithelial cancers.
Mutations involving EGFR could lead to its constant activation which could result in uncontrolled cell division – a predisposition for cancer.[8] Consequently, mutations of EGFR have been identified in several types of cancer, and it is the target of an expanding class of anticancer therapies.[2]
The identification of EGFR as an oncogene has led to the development of anticancer therapeutics directed against EGFR, including gefitinib[9] and erlotinib for lung cancer, and cetuximab for colon cancer.
Many therapeutic approaches are aimed at the EGFR. Cetuximab and panitumumab are examples of monoclonal antibody inhibitors. However the former is of the IgG1 type, the latter of the IgG2 type; consequences on antibody-dependent cellular cytotoxicity can be quite different.[10] Other monoclonals in clinical development are zalutumumab, nimotuzumab, and matuzumab. The monoclonal antibodies block the extracellular ligand binding domain. With the binding site blocked, signal molecules can no longer attach there and activate the tyrosine kinase.
Another method is using small molecules to inhibit the EGFR tyrosine kinase, which is on the cytoplasmic side of the receptor. Without kinase activity, EGFR is unable to activate itself, which is a prerequisite for binding of downstream adaptor proteins. Ostensibly by halting the signaling cascade in cells that rely on this pathway for growth, tumor proliferation and migration is diminished. Gefitinib, erlotinib, and lapatinib (mixed EGFR and ERBB2 inhibitor) are examples of small molecule kinase inhibitors.
There are several quantitative methods available that use protein phosphorylation detection to identify EGFR family inhibitors.[11]
[edit] EGFR and Lung Cancer
New drugs such as IRESSA and Tarceva directly target the EGFR. Patients have been divided into EGFR positive and negative, based upon whether a tissue test shows a mutation. EGFR positive patients have shown an impressive 60% response rate which exceeds the response rate for conventional chemotherapy.[12]
However many patients develop resistance. Two primary sources of resistance are the T790M Mutation and MET oncogene.[12] However, as of 2010 there was no consensus of an accepted approach to combat resistance nor FDA approval of a specific combination.
[edit] Preclinical
Efficient conversion of strongly absorbed light by plasmonic gold nanoparticles to heat energy and their easy bioconjugation suggest their use as selective photothermal agents in molecular cancer cell targeting. Two oral squamous carcinoma cell lines (HSC 313 and HOC 3 Clone 8) and one benign epithelial cell line (HaCaT) were incubated with anti-epithelial growth factor receptor (EGFR) antibody conjugated gold nanoparticles and then exposed to continuous visible argon ion laser at 514 nm. It is found that the malignant cells require less than half the laser energy to be killed than the benign cells after incubation with anti-EGFR antibody conjugated Au nanoparticles. No photothermal destruction is observed for all types of cells in the absence of nanoparticles at four times energy required to kill the malignant cells with anti-EGFR/Au conjugates bonded. Au nanoparticles thus offer a novel class of selective photothermal agents using a CW laser at low powers.[13]
[edit] Possible involvement in axonal regeneration
In 2005 it was shown that inhibitors of EGFR could enhance axonal regeneration on non-conducive substrates such as CNS myelin.[14] In July 2007 it was discovered that the blood clotting protein fibrinogen also activates EGFR, thereby inhibiting regeneration of axons.[15]
[edit] Natural EGFR inhibitors
Natural inhibitors include potato carboxypeptidase inhibitor (PCI), which contains a small cysteine-rich module, called a T-knot scaffold, that is shared by several different protein families, including the EGF family. Structural similarities with these factors can explain the antagonistic effect of PCI.[16]
[edit] Interactions
Erlotinib or Gefitinib for Lung Cancer
Clinical Therapeutics
Treatment of Non–Small-Cell Lung Cancer with Erlotinib or Gefitinib
V.D. Cataldo and Others
Advanced-stage non–small-cell lung cancer (NSCLC) is currently considered an incurable disease for which standard chemotherapy provides marginal improvement in overall survival at the expense of substantial morbidity and mortality. Even with the addition of newer agents, such as bevacizumab, to chemotherapy, the median overall survival of patients with metastatic NSCLC remains approximately 1 year.
Clinical Pearls
Which patients with non–small-cell lung cancer appear to benefit most substantially from treatment with erlotinib or gefitinib?
The available trial data suggest that EGFR tyrosine kinase inhibitors have efficacy that is similar to that of standard chemotherapy as second- or third-line treatment for patients with advanced NSCLC. Among patients receiving first-line therapy, tyrosine kinase inhibitors appear to be inferior to standard chemotherapy overall but superior for selected patients, especially for those with activating EGFR mutations.
For how long should treatment with erlotinib or gefitinib be continued?
Daily erlotinib or gefitinib therapy should be continued for as long as the patient's performance status is adequate and there is no clinical or radiographic progression, since patients with stable disease have been shown to derive clinical benefit. Furthermore, data support the continuation of treatment even if a loss of response is documented, since tumor progression is accelerated to a greater degree if the agent is discontinued.
Morning Report Questions
Q. Concurrent treatment with which medications should be avoided in patients being treated with erlotinib or gefitinib?
A. The solubility of both erlotinib and gefitinib is pH dependent. Agents that alter gastric pH, such as H2-receptor antagonists and proton-pump inhibitors, can substantially reduce the plasma levels of the EGFR tyrosine kinase inhibitors, and their concomitant use should be avoided.
Q. What is the most common dose-limiting adverse effect that limits erlotinib or gefitinib dosing?
A. In phase 1 studies of both agents, diarrhea was the dose-limiting effect. Diarrhea occurs in up to 55% of patients who are treated with erlotinib, with severe diarrhea occurring in 6% of patients. The incidence of diarrhea in patients receiving gefitinib ranges from 27 to 35%. Unlike traditional cytotoxic agents, erlotinib and gefitinib do not typically cause myelosuppression, neuropathy, alopecia, or severe nausea.
domingo, 6 de marzo de 2011
El topiramato o topamax muy usado en España...
Octavio Huerta de Mora posted in cibermedicos.Octavio Huerta de Mora4:41pm Mar 5
El topiramato o topamax muy usado en España tendría relación con defectos de nacimiento como labio leporino o paladar Hendido según nuevo informe de la FDA. A tomar en cuenta en sus prescripciones colegas!
Topiramate Linked to Birth Defects
topirol topamac topictal
www.medscape.com
: New human data show that the prevalence of cleft lips and palates in infants born to women taking topiramate was roughly 3 times higher than that for women taking other antiepileptic drugs
Topiramate Linked to Birth Defects
Robert Lowes
Posted: 03/04/2011
|
|
processing....
March 4, 2011 — Pregnant women taking topiramate (Topamax, Ortho-McNeil Janssen) to treat epileptic seizures or prevent migraine headaches have an increased risk of bearing children with a cleft lip or palate, the US Food and Drug Administration (FDA) announced today.
Consequently, clinicians should warn women of childbearing age about the possibility of these birth defects if they become pregnant while taking the medication.
"Health care professionals should carefully consider the benefits and risks of topiramate when prescribing it to women of childbearing age," said Russell Katz, MD, director of the Division of Neurology Products in the FDA's Center for Drug Evaluation and Research, in a statement from the FDA. "Alternative medications that have a lower risk of birth defects should be considered."
Pregnant women prescribed the medication should continue to take it unless advised not to by a clinician.
Topiramate, an anticonvulsant, is approved for treating certain seizures in patients with epilepsy and preventing migraine headaches. However, it is not indicated for treating the pain of such headaches when they occur. The drug also is used on an off-label basis to treat weight loss, alcohol dependence, and psychiatric illnesses such as bipolar disorder.
According to new data from the North American Antiepileptic Drug Pregnancy Registry, infants exposed to topiramate as a single therapy in the first trimester of pregnancy had a 1.4% prevalence of oral clefts compared with 0.38% to 0.55% for infants exposed to other antiepileptic drugs. The prevalence rate was even lower — 0.07% — for infants of mothers who did not have epilepsy and were not being treated with other AEDs.
These findings have prompted the agency to strengthen the label warning for topiramate by changing its pregnancy classification to category D. This category means that there are human data showing positive evidence of human fetal risk, but that the drug's benefits in pregnant women may outweigh the risks in some situations. The drug's pregnancy category previously had been a lower category C because of the absence of human data.
The FDA also will update the patient medication guide and prescribing information for the brand name and generic versions of topiramate.
More information about today's announcement is available on the FDA Web site.
To report adverse events related to topiramate, contact MedWatch, the FDA's safety information and adverse event reporting program, by telephone at 1-800-FDA-1088, by fax at 1-800-FDA-0178, online at http://www.fda.gov/medwatch or by mail to MedWatch, FDA, 5600 Fishers Lane, Rockville, Maryland 20852-9787.
Authors and Disclosures
Journalist
Robert Lowes
Freelance writer, St. Louis, Missouri
Disclosure: Robert L. Lowes has disclosed no relevant financial relationships.
 Print This
Print This  Email this
Email this -
 Share
Share
De: Octavio Huerta de Mora <notification+yfo6tzj9@facebookmail.com>
Fecha: 5 de marzo de 2011 16:41
Asunto: [cibermedicos] El topiramato o topamax muy usado en España...
Para: Claudio Mori Gonzales <clagui57@gmail.com>
| |||||||||